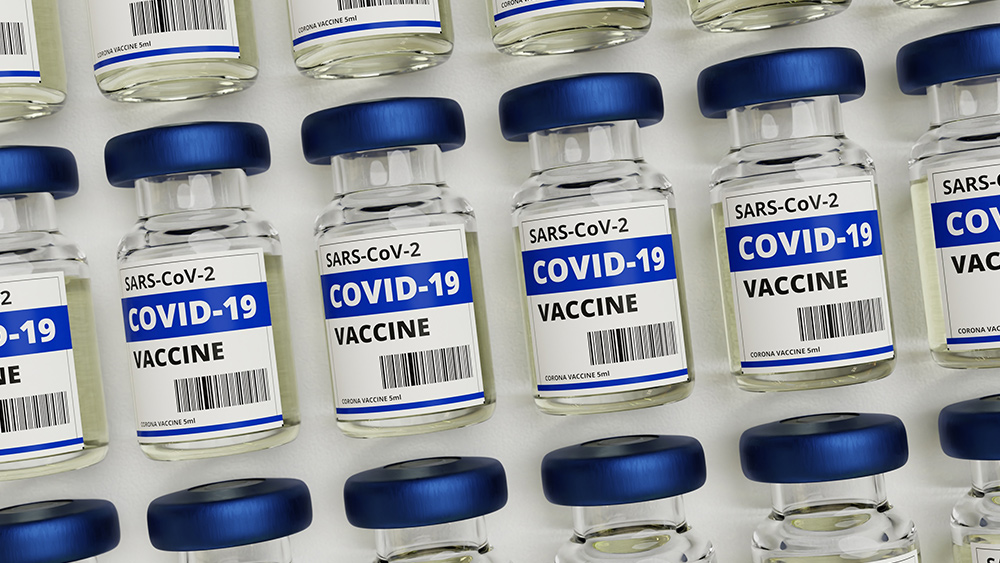
Some experts at the FDA voted against emergency-use authorization for Pfizer-BioNTech coronavirus vaccine
12/16/2020 / By Arsenio Toledo
Several vaccine experts in the Food and Drug Administration (FDA) have expressed concerns relating to the safety of the Wuhan coronavirus (COVID-19) vaccine jointly developed by Pfizer and BioNTech, voting against granting it emergency-use authorization.
On Thursday, the FDA’s Vaccines and Related Biological Products Advisory Committee met to vote on whether or not to recommend the Pfizer-BioNTech vaccine for emergency-use authorization. The committee discussed whether the evidence they had supported the view that the vaccine, known as BNT162b2, is effective in preventing people aged 16 and older from contracting the coronavirus.
Seventeen members of the committee voted in favor of granting the vaccine emergency-use authorization. Four voted against and one abstained.
The one abstention came from Dr. Cody Meissner, an expert on childhood vaccination and chief of pediatrics at Tufts Medical Center in Boston.
“I do not believe we have sufficient data for 16- and 17-year-olds,” he said during the committee’s meeting. Meissner argued that the vaccine should be withheld from use for individuals under the age of 18.
The four people who voted against were Dr. Archana Chatterjee, dean of the Chicago Medical School; Dr. David Kim, director of the Department of Health and Human Services’ vaccines division; Dr. Michael Kurilla, an expert on infectious diseases at the National Institutes of Health; and A. Oveta Fuller, a professor of microbiology and African studies at the University of Michigan.
Listen to this episode of the Health Ranger Report, a podcast by Mike Adams, the Health Ranger, as he talks about how the supposed second wave of coronavirus infections, hospitalizations and deaths is nothing more than a complete fabrication manipulated into existence by the mainstream media.
Concerns raised regarding use of vaccines on teenagers
The four voted against the emergency-use authorization due to the inclusion of teenagers into the authorization. They also stated that there has not been enough time to study the long-term effects of the coronavirus vaccine.
“My ‘no’ vote was because of the inclusion of 16- and 17-year-olds. Unfortunately, I and other members who had also voted ‘no’ did not have an opportunity to explain our positions before the meeting was adjourned,” said Kim in an email. “I would have voted ‘yes’ enthusiastically had the language been ‘…18 years of age and older.”
Chatterjee agreed. She said she was afraid that, since teenagers are not able to make their own decisions, their parents may force them to get the vaccine, believing it is safe for their kids to take.
“We have limited safety and efficacy data on how the vaccine affects the pediatric population,” she said.
Chatterjee also argued that since teenagers do not belong in a high-risk group, and because most of them will not be eligible to receive a vaccine soon anyway, they should be excluded from being given the vaccine so that researchers may gather more data.
“Unfortunately, there wasn’t an opportunity to explain my vote at the meeting,” said Chatterjee. “I considered voting to abstain, but thought it would be important for the FDA to make note of the concerns of those who voted ‘no.'”
Kurilla had similar concerns, stating that the amount of scrutiny that goes into approving an emergency-use authorization is not as thorough as a full FDA approval.
The emergency-use authorization is only supposed to be used for life-threatening conditions and ailments. Kurilla argues that this means only people with severe cases of COVID-19 – defined as having a high risk of mortality – should receive the treatment.
Kurilla believes that the general public might be confused regarding the distinction between full approval and emergency-use authorization. If the FDA continues with distribution, it needs to convey that more data is still being gathered about the vaccine. If the FDA is confident in the Pfizer-BioNTech vaccine, then they should have no problem communicating to the public that more needs to be done before full licensure.
Fuller’s main concern with the vaccine is that it has not been studied for long enough. The broad emergency-use authorization given to Pfizer-BioNTech’s vaccine is “premature” because there is not enough data to make her certain of its safety and efficacy.
She’s asking for several more months of “expanded access Phase 3 clinical study,” which would involve volunteer healthcare workers and residents of long-term care homes. This would satisfy the need for more evidence that can provide researchers with enough information regarding the supposed safety of the vaccine.
Pfizer-BioNTech vaccines to begin distribution within days of approval
Despite the protests put forward by the people on the advisory committee, the FDA has decided that it would grant emergency-use authorization to Pfizer-BioNTech. Following the authorization, the first shots are expected to be given within the next few days. The United States has already ordered around 2.9 million initial doses of the vaccine.
The first people to obtain the vaccine will be those categorized as “highly vulnerable individuals.” This includes senior citizens living in nursing homes and other long-term care facilities as well as healthcare workers such as doctors and nurses. (Related: UK begins mass vaccination program with Pfizer, starting with the elderly and healthcare workers.)
On top of the initial 2.9 million doses, the U.S. also plans to distribute a total of 25 million doses of the vaccine before the end of the year. Authorities are already warning most Americans that there is a strong chance they will not be one of the 12.5 million people who will get vaccinated immediately.
Given the pace of Pfizer-BioNTech’s production, the number of vaccine doses going to other countries and the large volume of the U.S’s order, the majority of Americans are not expected to get vaccinated until late into the first quarter or early in the second quarter of next year.
Learn more about the many vaccines being rushed out of development, such as Pfizer-BioNTech’s vaccine, by reading the latest articles at Vaccines.news.
Sources include:
Submit a correction >>
Tagged Under:
Big Pharma, BioNTech, coronavirus, coronavirus vaccine, covid-19, emergency use authorization, FDA, flu, harmful medicine, infections, outbreak, pandemic, Pfizer, superbugs, teenage vaccinations, vaccines, virus
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
BadDoctors.News is a fact-based public education website published by Bad Doctors News Features, LLC.
All content copyright © 2018 by Bad Doctors News Features, LLC.
Contact Us with Tips or Corrections
All trademarks, registered trademarks and servicemarks mentioned on this site are the property of their respective owners.